Ocean Newsletter
No.34 January 5, 2002
-
About Global Warming
Noriyuki NASU Professor Emeritus, The University of Tokyo
If global warming continues, the continental glacier will melt and bring a rise in sea level. Coastal and low-lying cities, harbors and croplands could submerge. This is the most terrifying outcome. Global warming should be prevented.
-
Rise in the Sea Level and Asia-Pacific Region
Nobuo MIMURA Professor of the Center for Water Environment Studies, Ibaraki University / Selected Papers No.3(p.6)
The report of the IPCC, an organization of the United Nations that assesses the scientific, technical and socio-economic information for the understanding of the risk of human-induced climate change, explains that warming exerts a great influence on the marine and coastal zones of the world. Serious repercussions such as inundation and flooding are expected in the Asia-Pacific region due to the series of sea level rise and flood tide.
Selected Papers No.3(p.6) -
Effects of Global Warming on Aquatic Resources
Tomoyasu KAWAI Exertive board member, Society for Fishery Industry in the 21st Century / Selected Papers No.3(p.8)
The Jomon period forewarns of global warming to a certain extent. Even then the conditions were not easy, and when global warming progresses in a short time, what will happen remains in the realm of the unknown. Then, the productivity of the sea will decrease for certain.
Selected Papers No.3(p.8) -
Stroring CO2 in Sunken Places on the Ocean Floor
Izuo AYA Manager, Osaka Branch Office, National Maritime Research Institute / Selected Papers No.3(p.10)
The vast ocean is a promising place to store huge amounts of recovered CO2 to mitigate global warming. The National Maritime Research Institute has conducted research on CO2 ocean sequestration technologies for the past 11 years and proposed the CO2 Sending Method for Ocean Storage, COSMOS.
Selected Papers No.3(p.10)
Stroring CO2 in Sunken Places on the Ocean Floor
The vast ocean is a promising place to store huge amounts of recovered CO2 to mitigate global warming. The National Maritime Research Institute has conducted research on CO2 ocean sequestration technologies for the past 11 years and proposed the CO2 Sending Method for Ocean Storage, COSMOS.
Deep-sea disposal of CO2 : an innovative technique to mitigate protecting the global warming
Carbon dioxide, or CO2, is generated from the combustion of fossil fuels, typically petroleum and natural gas. CO2 is a compound that has one carbon atom (C) and two oxygen atoms (O) combined. Because the oxygen atom is 1.33 times heavier than the carbon atom, the weight of CO2 generated by combustion is about three times as heavy as the unburnt fuel. Japan imports 800,000 tons of crude oil and other fossil fuels every day. This means that four oil tankers, each 200,000 tons in capacity, are required to transport them, and that to transport the CO2 that these fossil fuels discharge to the atmospheric air when combusted, ten oil tankers, each 200,000 tons in capacity, are required if the CO2 is to be loaded on these tankers in the form of liquid. If 5% of the CO2 can be recovered, it fits into one 200,000-ton oil tanker in every two days. Considering that the CO2 discharged in Japan is less than 5% of the total CO2 being discharged worldwide, we can understand how difficult it is to solve the global warming problem when viewed only from a technical standpoint. The situation seems more aggravating if we consider the facts: China and other developing countries that consume enormous energies now and in the future are not members in the Kyoto Protocol, and the population explosion is happening in India and Africa.
The number of molecules at the average depth of 3,795 m in the sea is 430 times as large as in atmospheric air. In 1978 American scientists proposed the idea for controlling the climate by disposing of CO2 in the depths of the ocean. This idea did not attract much attention. However, it was reconsidered after a meteorologist presented evidence on global warming in the U.S. Upper House in 1988. In Japan a deep-sea CO2 disposal project began in 1990: the first official research project of this kind in the world. In December 1997 the Third Conference of Parties of the United Nations Framework Convention on Climate Change (COP3) was held in Kyoto. In 1998 the deep-sea disposal of CO2 was added to the Outline of the Battle against Global Warming as one innovative technique to mitigate the global warming.
There is an increasing awareness that the deep-sea disposal of CO2 must be studied as one of the few techniques that enable us to dispose of environment-disrupting substances in huge quantities. Research and experiments concerning the deep-sea CO2 disposal are now expanding worldwide as the U.S., Norway and Canada have begun to do research and conduct experiments.
Various methods of disposing of CO2 in the sea have until now been proposed. They are broadly classified into a dissolution method and a storage method. With the dissolution method, recovered CO2 in liquid or gas form is dissolved and diffused at a depth of 2,000 m or shallower in the sea; this method takes advantage of the immense capacity of the ocean. Its working principle is that the recovered CO2 is integrated into the natural circulation process in which part of the excess CO2 in atmospheric air dissolves in the sea. With the storage method, recovered CO2 in liquid form is stored in sunken places 3,500 m or deeper on the ocean floor where CO2 becomes heavier than the seawater with CO2 dissolved. This method aims to keep the effects of stored CO2 on the oceanic environment to a minimum. Because both methods are technically viable, the effects on the marine environment and the ecosystem must be evaluated before either method is adopted for disposing of CO2. Table 1 shows a summary of the features and differences of the two methods. Although the dissolution method is superior to the storage method in technical viability and cost because of the shallow disposal depth, the storage method gains an overwhelming advantage over the dissolution method if we consider the storage period and reversibility (whether the situation can be reversed to the original situation), as well as the precision and ease of environmental impact assessment.
With all this considered, I conclude that the storage method should be used to dispose of CO2 in the deep sea and that the technical development, including the assessment of impact on the ecosystem in and around the CO2 storage site, should be undertaken.
Research on the deep-sea disposal of CO2 at the National Maritime Research Institute
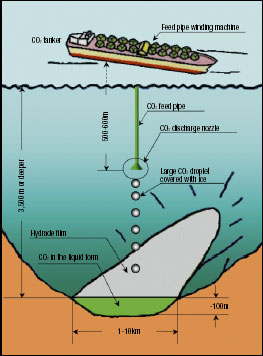 Fig.1 Concept of COSMOS
Fig.1 Concept of COSMOS
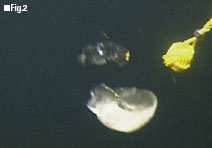 A slurry of low-temperature CO2 (8 cm in diameter) is sinking by its own weight at a depth of 530 m in the sea.
A slurry of low-temperature CO2 (8 cm in diameter) is sinking by its own weight at a depth of 530 m in the sea.
We at the National Maritime Research Institute (former Ship Research Institute) have conducted research on the deep-sea disposal of CO2 for the past 11 years, assuming that the storage method is adopted. We clarified the properties of the CO2 hydrate forming at depths 500 m or deeper (at a point in the northern sea of the Pacific Ocean) and 900 m (at a point in the northern sea of the Atlantic Ocean) and proposed the COSMOS (CO2 Sending Method for Ocean Storage) method, which is designed to overcome the drawbacks of the storage method: the difficulty and high cost in transporting CO2 to the depths of the sea. (The CO2 hydrate is a metastable crystalline compound with properties identical to those of ice, and it turns into a sherbet when it mixes with water.)
The net cost of deep-sea storage of CO2 becomes nearly equal to that of the dissolution method if the COSMOS is used, specifically it is estimated to be about 20% of overall power generation cost. Considering that Japan must reduce CO2 as specified in the Kyoto Protocol (minus 6% of the result accomplished in 1990) and implement the reduction activities specified in the Energy Saving Law and other laws and regulations, the amount of CO2 to be reduced by the deep-sea CO2 disposal is estimated to be about 5% at most. Therefore, the cost of the deep-sea storage of CO2 is about 1% of the overall power generation cost, which is at an allowable level.
Figure 1 shows the concept of the COSMOS. CO2 to be transported by a tanker is cooled to around minus 55 Celsius, where it is just about to turn into dry ice, so that the pressure on the tank can be reduced as much as possible. This low-temperature CO2 becomes sufficiently heavier than seawater at a depth of 500 m. If it can be discharged as a large droplet of one meter or larger in diameter, it will sink by its own weight to the storage site 3,500 m or deeper in the depths of the sea without being affected by the heat from seawater or the buoyant force from ice layers which cover the liquid bubble. Figure 2 shows a low-temperature CO2 slurry of 8 cm in diameter sinking by its own weight toward a storage site at the speed of 0.3 m/s around the depth of 530 m in the sea, which was photographed in the field experiment jointly conducted with the Monterey Bay Aquarium Research Institute (MBARI). The National Maritime Research Institute with the cooperation of the MBARI plans to begin the work of the COSMOS development using a large high-pressure tank for operations at a depth of 6,000 m. This tank will be completed soon.
| Dissolution method | Storage method | |
| Technical viability | ○ CO2 to be discharged at a depth of 2,000 m or shallower | △ CO2 to be discharged at a depth of 3,500 m or shallower |
| Cost | ○ | △ ○ if COSMOS is activated |
| Storage period | 50 to 200 years The period depends on the depth and the sea area | Longer than 2000 years A period longer than the ocean's vertical circulation cycle can be desired |
| Reversibility (presence or absence of a safety valve) | × Basically irreversible because the mothod is based on an irreversible process | ○ CO2 can be retrieved, though the work of retrieval involves cost |
| Environmental impact | Difficult to clarify the level of environmental impact because 0 × ∞ | Possible to clarify the level of environmental impact because of finite × finite |
