Ocean Newsletter
No.576 August 5, 2024
-
Mineralization and Decarbonization of Marine Organisms
SUZUKI Michio (Professor, Graduate School of Agriculture and Life Sciences, University of Tokyo)
The main component of limestone is calcium carbonate, which is produced by marine organisms such as shellfish and corals through a biological reaction called "biomineralization." If we can elucidate the mechanism of this biomineralization and develop technology to efficiently synthesize calcium carbonate, we can contribute to reducing the excessive concentration of carbon dioxide in the atmosphere.
-
Approaches to Coral Reef Conservation and Restoration that Incorporate Ecological Knowledge
SHIKINA Shinya (Director, Institute of Marine Environment and Ecology, National Taiwan Ocean University)
Coral reefs are ecosystems that nurture a wide variety of marine life. They also provide us with many blessings. In recent years, coral propagation and planting projects have become popular in order to restore declining coral reefs. In this paper, we summarize the issues facing current coral reef conservation and restoration projects, and propose a new approach to conservation and restoration projects that incorporates basic knowledge from biology and ecology into the existing framework.
-
Kelp Forests, Uninomics, and Ecosystem Restoration Initiatives
TAKEDA Brian Tsuyoshi (Uninomics Kabushiki Kaisha)
The business model of Uninomics Kabushiki Kaisha. is to help restore kelp ecosystems by harvesting overgrazing urchins that cause coastal denudation, and culture them into uni, or sea urchin roe, for consumption. This article describes the details of the business, how obtaining J Blue Credits has provided them with a strong foundation, and the company’s expectations regarding J Blue Credits in the future.
Mineralization and Decarbonization of Marine Organisms
KEYWORDS
Biomineralization / Calcium Carbonate / Calcification
SUZUKI Michio (Professor, Graduate School of Agriculture and Life Sciences, University of Tokyo)
The main component of limestone is calcium carbonate, which is produced by marine organisms such as shellfish and corals through a biological reaction called “biomineralization.” If we can elucidate the mechanism of this biomineralization and develop technology to efficiently synthesize calcium carbonate, we can contribute to reducing the excessive concentration of carbon dioxide in the atmosphere.
What is Biomineralization?
Biomineralization refers to the phenomenon where organisms form shells, coral skeletons, and exoskeletons inside or outside their bodies. It is known that biomineralization deposits various minerals like silica, iron, and calcium. The most abundant mineral in terms of biomass on Earth is calcium carbonate, which is formed by various marine organisms.
Calcium carbonate probably brings to mind images of white powder or the columnar or rhombohedral crystals displayed at mineral exhibitions. However, the production of calcium carbonate in these organisms is strictly controlled in terms of crystal shape, size, polymorphism, and orientation. When observing the shells under a microscope, you can see the fine beautiful mictostructure. (Figure 1). Reproducing and manufacturing calcium carbonate with these characteristics artificially at room temperature and pressure is extremely difficult. The formation of calcium carbonate by organisms is believed not to be a simple inorganic chemical reaction. Instead, it is thought to be controlled by the involvement of special organic molecules.
To date, many substrate proteins and organic molecules related to biomineralization have been identified, including carbonic anhydrase, which converts carbon dioxide into bicarbonate ions; tyrosinase, which promotes the bonding of organic materials; chitinase, which refines chitin found in the exoskeletons of shrimp, crabs, and insects; and Pif family proteins, which have many negatively charged acidic amino acids and bind strongly to calcium carbonate. These are just some of the various new factors and reactions that have been revealed to be involved. In other words, the hybrid structure of organic and inorganic components is the greatest characteristic of calcium carbonate formed through biomineralization.
Calcium carbonate probably brings to mind images of white powder or the columnar or rhombohedral crystals displayed at mineral exhibitions. However, the production of calcium carbonate in these organisms is strictly controlled in terms of crystal shape, size, polymorphism, and orientation. When observing the shells under a microscope, you can see the fine beautiful mictostructure. (Figure 1). Reproducing and manufacturing calcium carbonate with these characteristics artificially at room temperature and pressure is extremely difficult. The formation of calcium carbonate by organisms is believed not to be a simple inorganic chemical reaction. Instead, it is thought to be controlled by the involvement of special organic molecules.
To date, many substrate proteins and organic molecules related to biomineralization have been identified, including carbonic anhydrase, which converts carbon dioxide into bicarbonate ions; tyrosinase, which promotes the bonding of organic materials; chitinase, which refines chitin found in the exoskeletons of shrimp, crabs, and insects; and Pif family proteins, which have many negatively charged acidic amino acids and bind strongly to calcium carbonate. These are just some of the various new factors and reactions that have been revealed to be involved. In other words, the hybrid structure of organic and inorganic components is the greatest characteristic of calcium carbonate formed through biomineralization.
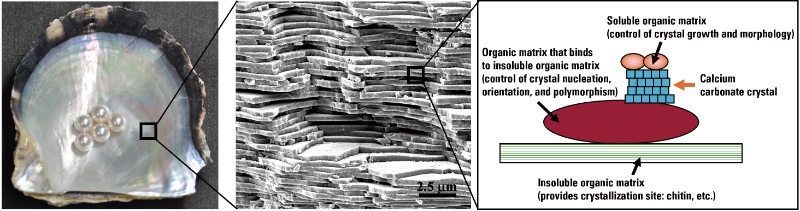
■ Figure 1 Microstructural Layer of Shell Calcium Carbonate
Left: Pearl and Akoya pearl oyster shell
Center: Microstructure of nacre
Right: Schematic diagram of calcium carbonate and organic matrix
Center: Microstructure of nacre
Right: Schematic diagram of calcium carbonate and organic matrix
A Hypothesis of the Carbon Cycle and Biomineralization Research
As the atmospheric carbon dioxide concentration increases from human activities and global warming becomes a problem, there is strong demand in not only in Japan but around the world to realize a sustainable decarbonized society. Research to accelerate decarbonization, such as direct underground storage of atmospheric carbon dioxide (CCS)1, artificial photosynthesis, and the use of hydrogen energy, is actively being conducted. The Earth's crust contains large amounts of carbon as coal and oil, but an equal or greater amount of carbon is said to be found in limestone, accounting for a large proportion of the total. Most of this limestone (about 40 million Gt) is believed to be derived from biomineralization by organisms. Compared to atmospheric carbon dioxide (760 Gt), the amount of carbon in limestone is overwhelmingly large, and it is easy to speculate that even a slight fluctuation in the amount of limestone would significantly affect atmospheric carbon dioxide concentrations. However, efforts to fix carbon dioxide into solid calcium carbonate have barely begun to be examined.
The background to this was the recognition that calcium carbonate production by marine biomineralization is a reaction that releases carbon dioxide from the ocean and does not contribute to carbon fixation. This theory was established before the 1990s, assuming that biomineralization was the same as inorganic calcium carbonate production reactions.
The background to this was the recognition that calcium carbonate production by marine biomineralization is a reaction that releases carbon dioxide from the ocean and does not contribute to carbon fixation. This theory was established before the 1990s, assuming that biomineralization was the same as inorganic calcium carbonate production reactions.
Potential to Control Carbon Dioxide Concentration
Due to this hypothesis, research to apply the results of biomineralization research to carbon dioxide fixation has not been conducted, but the situation is changing. Recent studies on biomineralization have revealed several new insights: the crystal polymorphism, morphology, orientation, and defects of calcium carbonate are strictly controlled; a rapid pH increase occurs before the calcification reaction in calcification sites within the organism; and special biomineral proteins act as catalysts to bind calcium and carbonate ions, making the process highly favorable from a kinetic standpoint. This suggests that in biomineralization, organisms remove hydrogen ions (protons) through metabolic reactions (Figure 2). I believe that if humans could appropriately handle protons by mimicking this process, carbon fixation through calcium carbonate production using calcium sources from seawater could become possible. The reaction from carbon dioxide to calcium carbonate is an exothermic reaction that proceeds without the need for external energy despite being entropically unfavorable. Additionally, the fixation cost is low, and it is stable as a solid at room temperature and pressure, so no energy cost is required for storage. Furthermore, the synthesized calcium carbonate can be utilized industrially (through CCUS)2, making it possible to sell and profit from it. Therefore, it offers numerous advantages (Figure 3).
Humanity has consumed large amounts of limestone for various industries and continues to emit large amounts of carbon dioxide. This carbon dioxide is returned to limestone through biomineralization by marine organisms. By researching the mechanisms of biomineralization and utilizing its outcomes, we aim to develop technology to efficiently synthesize calcium carbonate and enable human society to control atmospheric carbon dioxide concentrations freely.
Humanity has consumed large amounts of limestone for various industries and continues to emit large amounts of carbon dioxide. This carbon dioxide is returned to limestone through biomineralization by marine organisms. By researching the mechanisms of biomineralization and utilizing its outcomes, we aim to develop technology to efficiently synthesize calcium carbonate and enable human society to control atmospheric carbon dioxide concentrations freely.
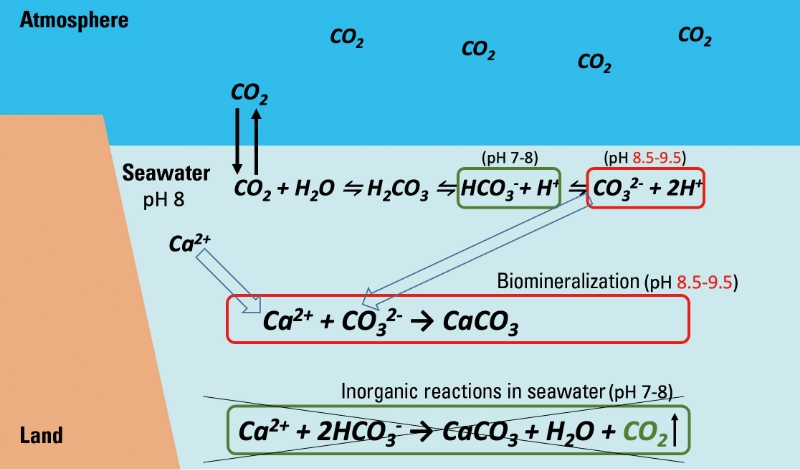
■ Figure 2 Differences Between Inorganic Reactions in Seawater and Biomineralization in Organisms
Top equation: Carbon dioxide dissolves and dissociates into bicarbonate ions and hydrogen ions (protons), leading to ocean acidification. In seawater at pH 8, it mainly exists as bicarbonate ions, but some protons further dissociate to form carbonate ions.
Middle equation: Calcification by marine organisms. Refer to the article.
Bottom equation: Inorganic calcification. Along with the generation of calcium carbonate, protons are generated from bicarbonate ions causing acidification, and protons react with bicarbonate ions dissolved in seawater to generate carbon dioxide. Traditionally, biological calcification was also thought to be this same reaction, which generates protons and carbon dioxide.
Middle equation: Calcification by marine organisms. Refer to the article.
Bottom equation: Inorganic calcification. Along with the generation of calcium carbonate, protons are generated from bicarbonate ions causing acidification, and protons react with bicarbonate ions dissolved in seawater to generate carbon dioxide. Traditionally, biological calcification was also thought to be this same reaction, which generates protons and carbon dioxide.
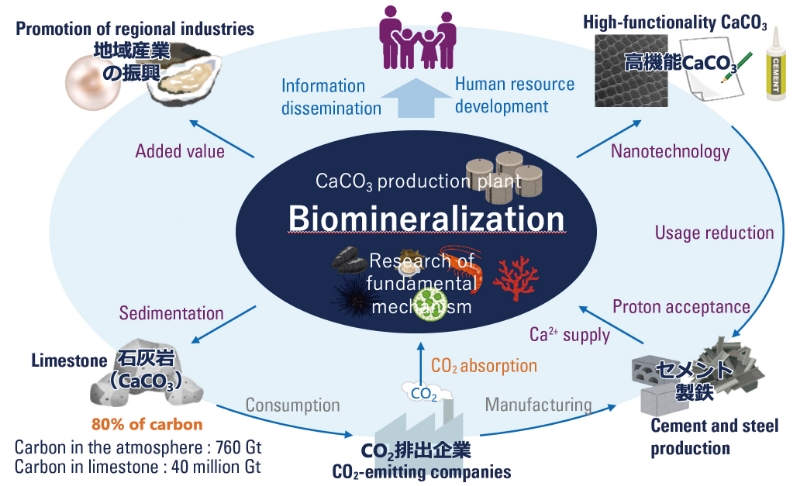
■ Figure 3 Decarbonization Cycle Centered on Biomineralization
1. Abbreviation for Carbon dioxide Capture and Storage. A technology to separate and capture carbon dioxide contained in exhaust gases from thermal power plants and factories and store it in stable underground strata.
2. Abbreviation for Carbon dioxide Capture, Utilization, and Storage. A technology not only to store captured carbon dioxide but also to effectively use it as a resource for crop production and chemical product manufacturing. Calcium carbonate is used in many applications, including cement, steel, papermaking, plastic plasticizers, cosmetics, and food.
2. Abbreviation for Carbon dioxide Capture, Utilization, and Storage. A technology not only to store captured carbon dioxide but also to effectively use it as a resource for crop production and chemical product manufacturing. Calcium carbonate is used in many applications, including cement, steel, papermaking, plastic plasticizers, cosmetics, and food.
